
But this is not the only effect we have to take into account. Thus, the attraction to the nucleus is weaker and the energy associated with the orbital is higher (less stabilized). As the principal quantum number, n, increases, the size of the orbital increases and the electrons spend more time farther from the nucleus. The filling order is based on observed experimental results, and has been confirmed by theoretical calculations. Thus, many students find it confusing that, for example, the 5 p orbitals fill immediately after the 4 d, and immediately before the 6 s. Generalized energy-level diagram for atomic orbitals in an atom with two or more electrons (not to scale).Įlectrons in successive atoms on the periodic table tend to fill low-energy orbitals first.

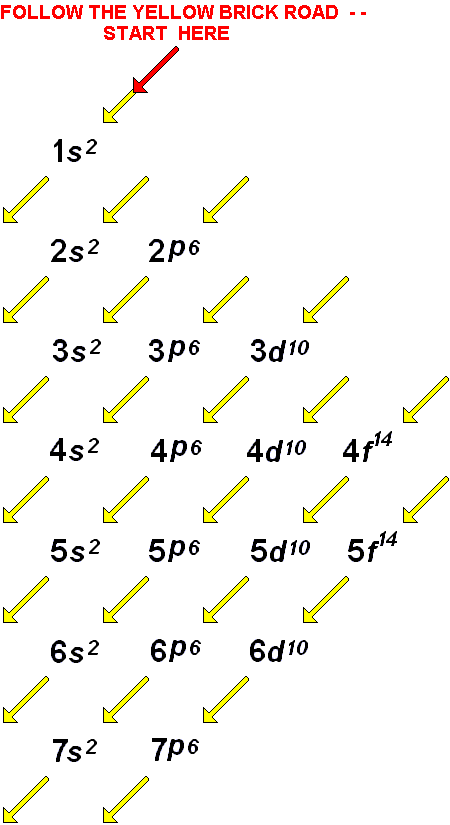
Such overlaps continue to occur frequently as we move up the chart. The 3 d orbital is higher in energy than the 4 s orbital. However, this pattern does not hold for larger atoms. The energy increases as we move up to the 2 s and then 2 p, 3 s, and 3 p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms. The 1 s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. Figure 11.1 depicts how these two trends in increasing energy relate. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different values of l differ so that the energy of the orbitals increases within a shell in the order s < p < d < f.

The energy of atomic orbitals increases as the principal quantum number, n, increases. The specific arrangement of electrons in orbitals of an atom determines many of the chemical properties of that atom. This allows us to determine which orbitals are occupied by electrons in each atom. Having introduced the basics of atomic structure and quantum mechanics, we can use our understanding of quantum numbers to determine how atomic orbitals relate to one another. Relate electron configurations to element classifications in the periodic table.Identify and explain exceptions to predicted electron configurations for atoms and ions.

Derive the predicted ground-state electron configurations of atoms.The periodic table can be divided into three categories based on the orbital in which the last electron to be added is placed: main group elements (s and p orbitals), transition elements (d orbitals), and inner transition elements (f orbitals).īy the end of this section, you will be able to: There are some exceptions to the predicted filling order, particularly when half-filled or completely filled orbitals can be formed. In the periodic table, elements with analogous valence electron configurations usually occur within the same group. Electron configurations and orbital diagrams can be determined by applying the Pauli exclusion principle (no two electrons can have the same set of four quantum numbers) and Hund’s rule (whenever possible, electrons retain unpaired spins in degenerate orbitals).Įlectrons in the outermost orbitals, called valence electrons, are responsible for most of the chemical behavior of elements. The relative energy of the subshells determine the order in which atomic orbitals are filled (1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, and so on).


 0 kommentar(er)
0 kommentar(er)
